
A simple way to understand and memorise the valency of first 20 elements of the periodic table
First 20 Elements The first 20 elements of the periodic table have been tabulated below, along with their symbols and atomic numbers. Learn more ⇒ Interactive Periodic Table Table of Contents What Information does the Atomic Number of an Element Provide? Why is Potassium denoted by the symbol 'K' and Sodium by the symbol 'Na'? Recommended Videos

7 Images Periodic Table With Names And Atomic Mass Number Valency And Review Alqu Blog
The valence (or valency) of an element is a measure of its combining power with other atoms when it forms chemical compounds or molecules. The concept of valence was developed in the last half of the 19th century and was successful in explaining the molecular structure of many organic compounds. The quest for the underlying causes of valence.
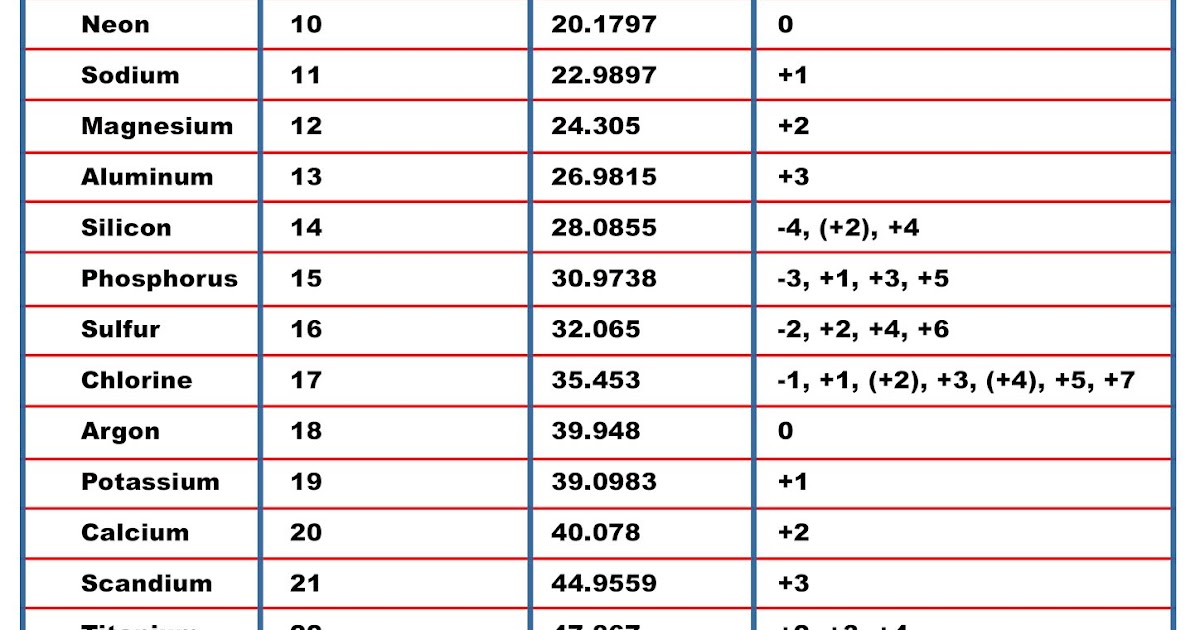
Periodic Table And Their Valency
Solution Valency: "The electrons present in the outermost shell of an atom are known as the valence electrons." "Valency is the combining capacity of an atom." The valency and valence electrons for the first 20 elements are discussed below: Suggest Corrections 953 Similar questions

valency trick how to find valency of elementsfirst 20 elements YouTube
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
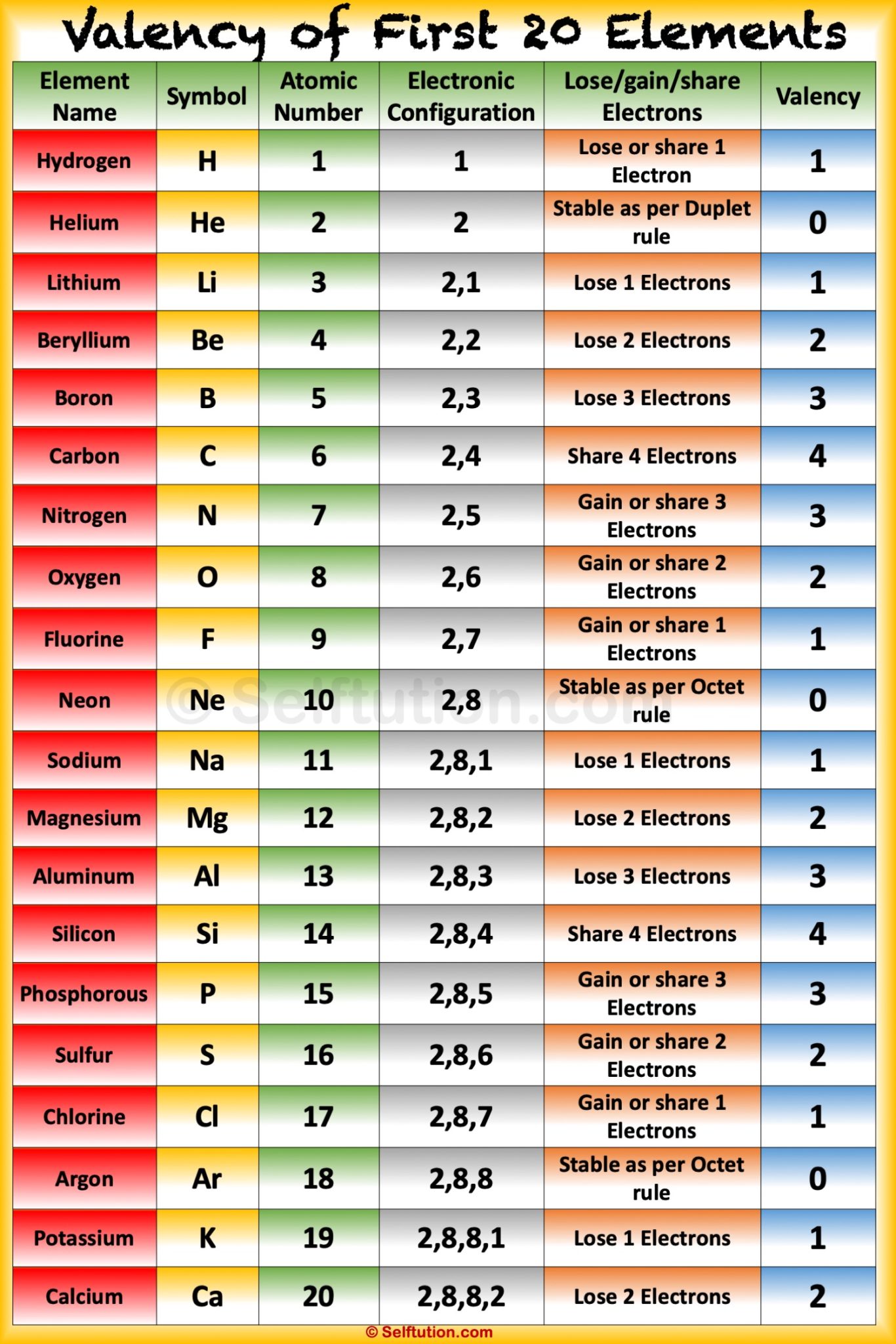
Valency and Variable Valency Valence Shell and Electrons » Selftution
So you're 2,8,8,2 rule looks like it shows the maximum amount of valence electrons elements can have in a period up to atomic number 20 (calcium). So elements in period 1 (hydrogen and helium) need 2 electrons to fill their valence shell. Elements in period 2 (building on the previous 2 electrons) need 8 more electrons to fill the second.

Electronic Configuration Definition, Shell, Position
What is the reason for this particular formula? The answer to the above question is "Valency". Let us know more about Valency and how it helps in determining a formula! Suggested Videos What is Valency? Valency is the measure of the combining capacity of atoms or molecules.

1 to 30 elements with valency electrons Brainly.in
1 Answer Stefan V. Jan 13, 2015 So, the first 20 elements in the periodic table start with H and end with Ca. The quickest way to remember the number of valence electrons is to form a relationship with the number of the group the element is located in.

valency table Scribd india
Q1What are the first 20 elements in order?H - HydrogenHe - HeliumLi - LithiumBe - BerylliumB - BoronC - CarbonN - NitrogenO - OxygenF - FluorineNe - NeonNa -.

chemistry first 20 elements table with electronic configuration and valency Brainly.in
Hence we assign a valence of 1 to H and to Cl. The valence of O is twice as great, and so we assign a value of 2. Example 4.4.1 4.4. 1: Formula Predictions. Use the data in the first table to predict what formula would be expected for a compound containing (a) sodium and fluorine; (b) calcium and fluorine.

20 elements name symbol. valency atomic mass atomic number Brainly.in
Solution Valency:- Valency is defined as the property of an element in which it has the combining capacity with another atom of an element. It is related to electrons present in their outer orbital. Valency can be determined by their respective groups in the periodic table. Determination of valency of first 20 elements:-
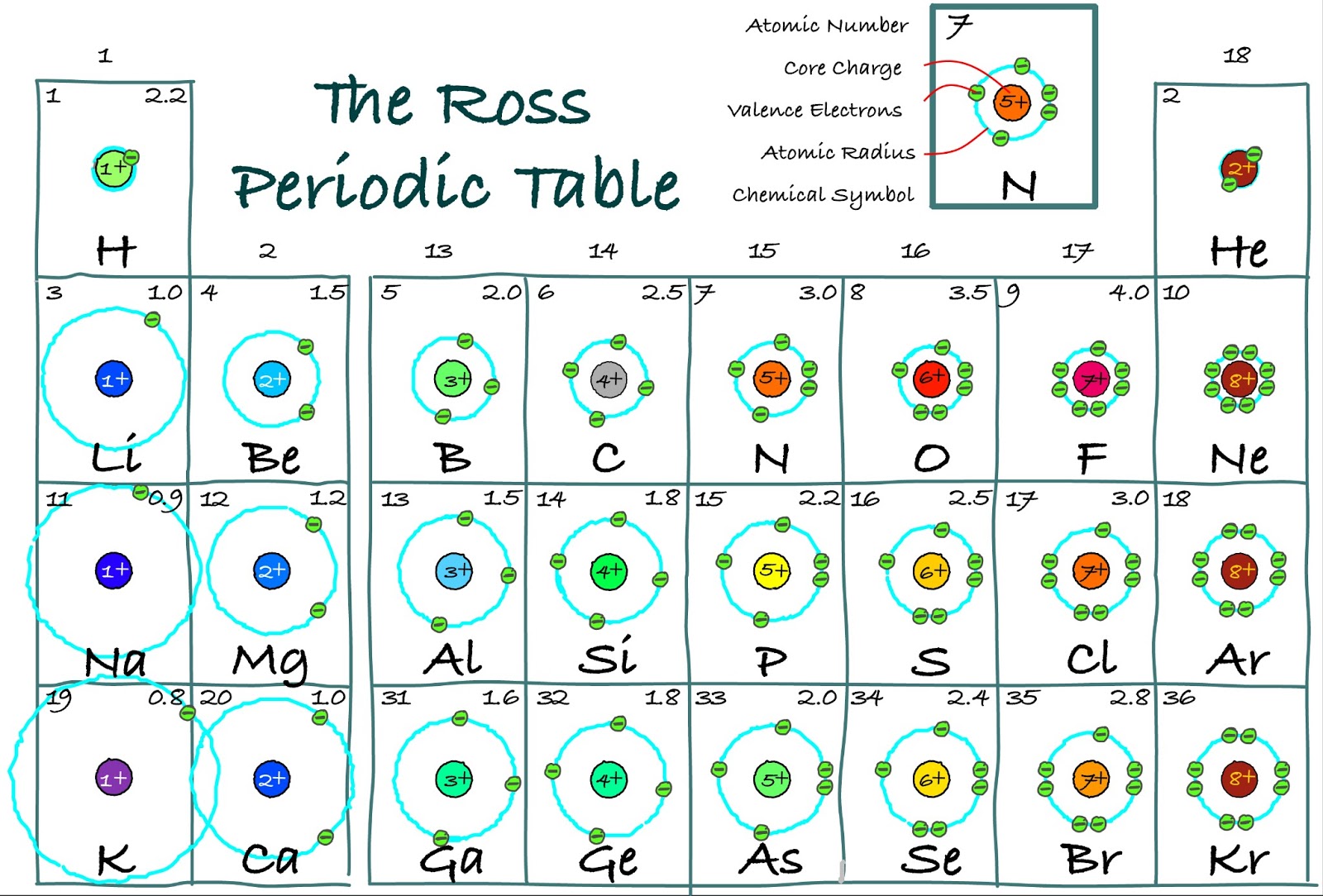
The Ross Periodic Table Core Charge Its Periodicity Across the Table
Updated on September 30, 2018. The words valence and valency have two related meanings in chemistry. Valence describes how easily an atom or radical can combine with other chemical species. This is determined based on the number of electrons that would be added, lost, or shared if it reacts with other atoms. Valence is denoted using a positive.
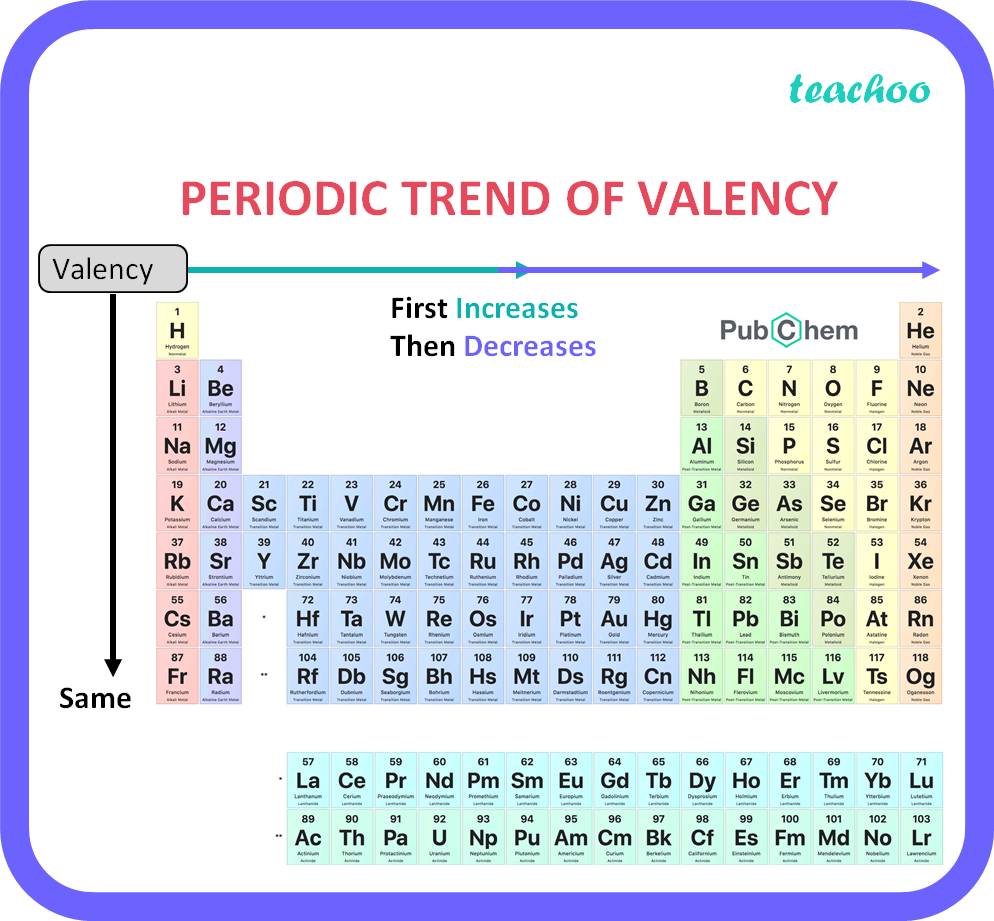
How does valency of an element vary across a period Class 10 Teachoo
Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell. Table of Element Valences Sources

Atomic number,mass number and valency of elements 1 to 30 YouTube
The valency of elements from 1 to 20 showcases some fascinating chemical behavior. Here are the valencies of selected elements: Hydrogen (H): Hydrogen typically exhibits a valency of +1, forming compounds like H2O (water) and HCl (hydrochloric acid).

what is the valency of first 20 elements ? Brainly.in
The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined - 4s 2, 4p 1). The completely filled d orbitals count as core, not valence, electrons. Transition elements or.

Name of Elements Symbol and Valency 1 to 20 Elements Chemistry YouTube
In less than 2 minutes, you'll remember the valency of elements 1 - 30! We'll use 2 valency tricks - valency for the first 20 elements and valency for elemen.

periodic table of elements printable flashcards chemistry etsy find various types of valency
Description The valence is the combining capacity of an atom of a given element, determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1.