
Potassium Facts
It is a very soft metal and can even be cut into pieces using a knife. It is silvery-white in appearance and is known to react vigorously with water. Potassium ions are known to play a vital role in our body such as nerve transmission and deficiency of potassium may result in abnormal heart rhythms.
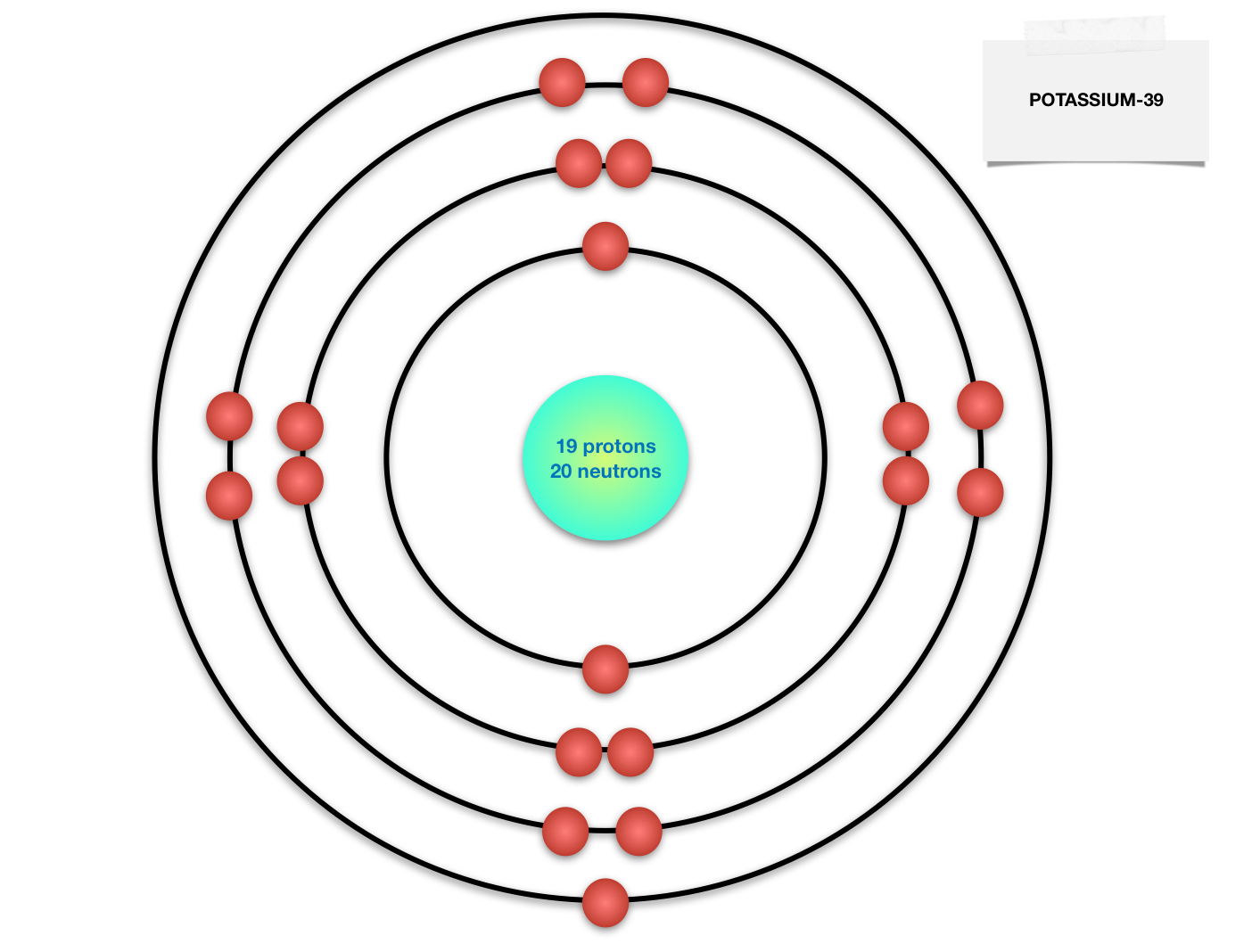
Bohr Diagram For Potassium
How to draw the Bohr-Rutherford Diagram for Potassium. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on.
Potassium Bohr Model
2.8: Summary of Bohr's Contribution. Page ID. David M. Hanson, Erica Harvey, Robert Sweeney, Theresa Julia Zielinski. Chemical Education Digital Library (ChemEd DL) Bohr's proposal explained the hydrogen atom spectrum, the origin of the Rydberg formula, and the value of the Rydberg constant. Specifically it demonstrated that the integers in.
Periodic Table Potassium Protons Neutrons Electrons
What is the Bohr model of potassium? Chemistry Matter Basic Atomic Structure 1 Answer MathFact-orials.blogspot.com May 31, 2016 Please see the diagram below: Explanation: Answer link Please see the diagram below:
Atomic Structure (Bohr Model) for Potassium (K) YouTube
1 λ = − ℜ( 1 n2 2 − 1 n2 1) Except for the negative sign, this is the same equation that Rydberg obtained experimentally. The negative sign in Equation 2.6.5 and Equation 2.6.6 indicates that energy is released as the electron moves from orbit n2 to orbit n1 because orbit n2 is at a higher energy than orbit n1.
Potassium Electron Configuration Full Potassium Electron
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.
Bohr Model For Potassium
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).

Potassium Facts, Symbol, Discovery, Properties, Uses
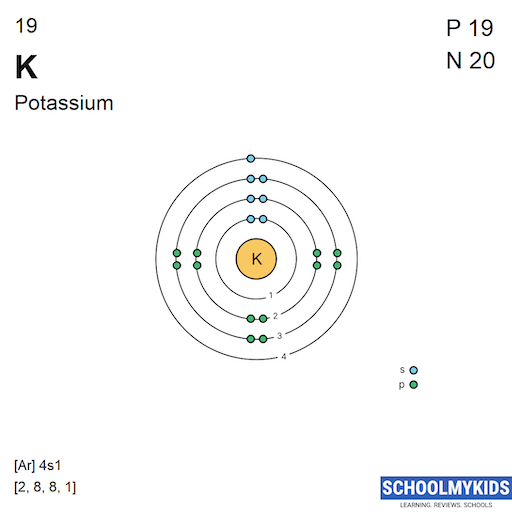
Here are a few highlights that you learned in the last section about Bohr's model:. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s subshell and calcium has an electron configuration of [Ar]4s 2. This gives calcium an outer-shell electron configuration corresponding.

What is the Bohr model for potassium? Quizlet
In this video we'll look at the atomic structure and Bohr model for the Potassium atom (K). We'll use a Bohr diagram to visually represent where the electron.

Potassium electron configuration. Illustration of the atomic structure
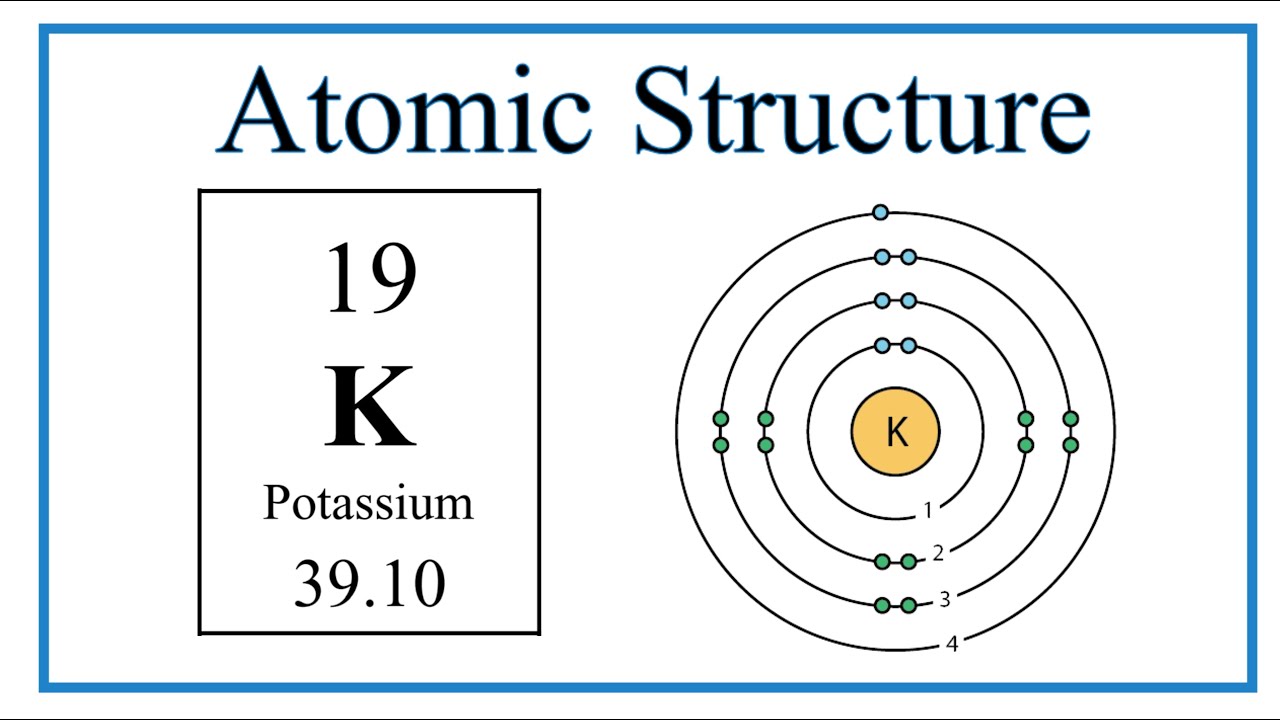
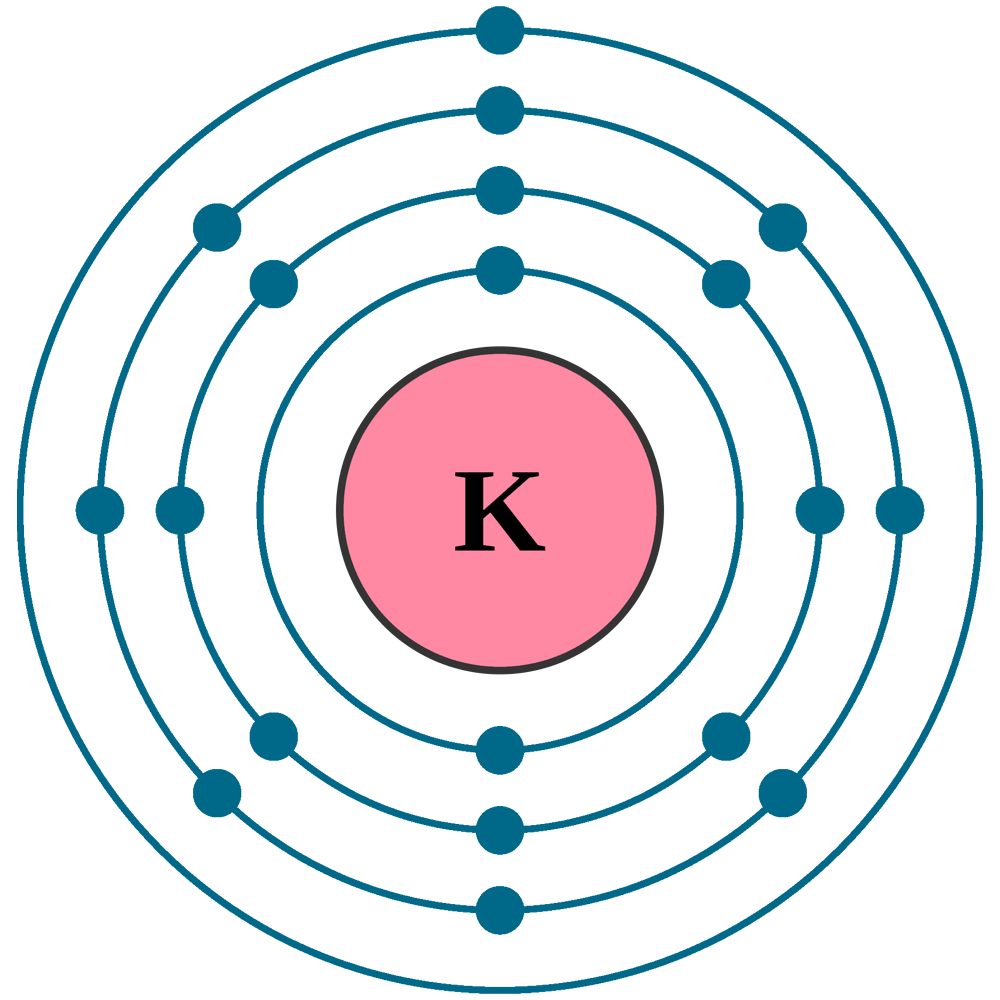
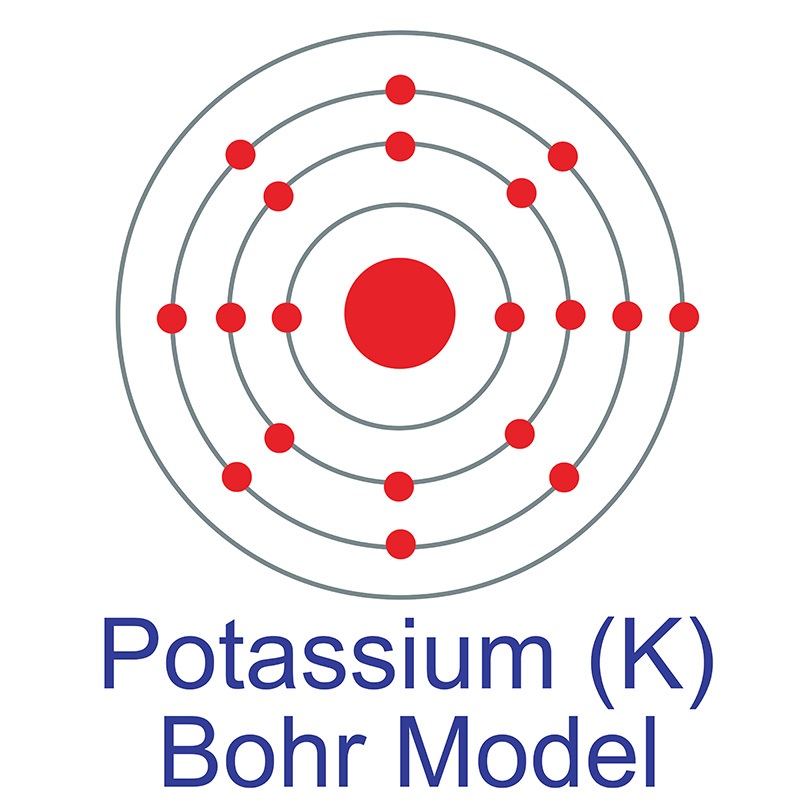
The Bohr Model of Potassium (K) has a nucleus that contains 20 neutrons and 19 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Potassium contains only 1 electron that also called valence electron. Page Contents show

Potassium Bohr Model Diagram, Steps To Draw Techiescientist
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Optical parameters and dispersion behavior of potassium magnesium
Potassium (K) atom electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Electron configuration of potassium through orbit Scientist Niels Bohr was the first to give an idea of the atom's orbit.
Electron arrangements
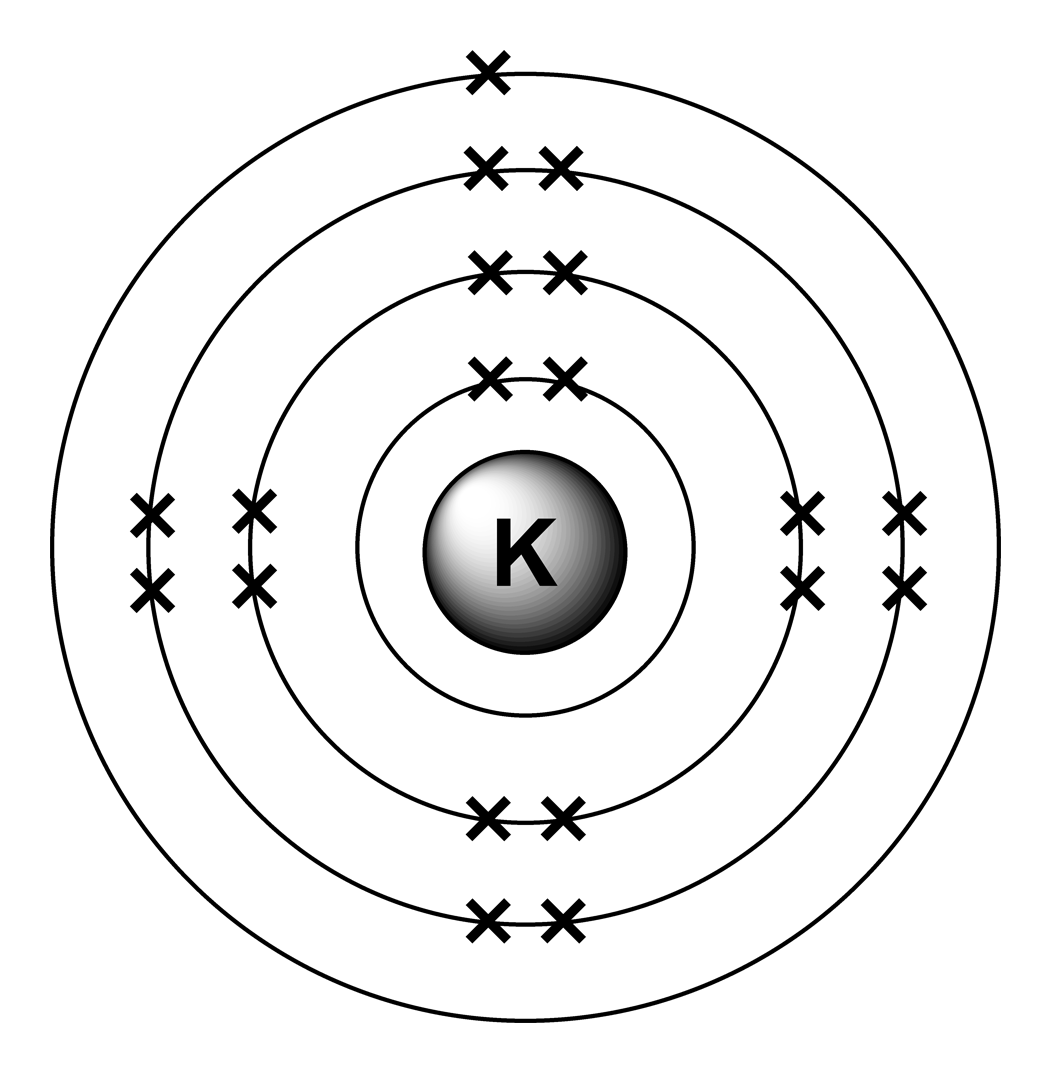
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus. This is sometimes called the Bohr, or the 'solar system', model. Download this

Potassium Periodic Table Protons Neutrons And Electrons Awesome Home
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?
/potassiumatom-58b602485f9b5860464c55ea.jpg)
Atoms Diagrams Electron Configurations of Elements
The melting point of potassium is 63.5 °C and its boiling point is 759 °C. Potassium has many isotopes, but out of them 39 K has an abundance of around 93%. Density of potassium is less than the density of water. Hence it floats on water. Potassium is the 2nd lightest metal after lithium.

3d render of atom structure of potassium isolated over white background
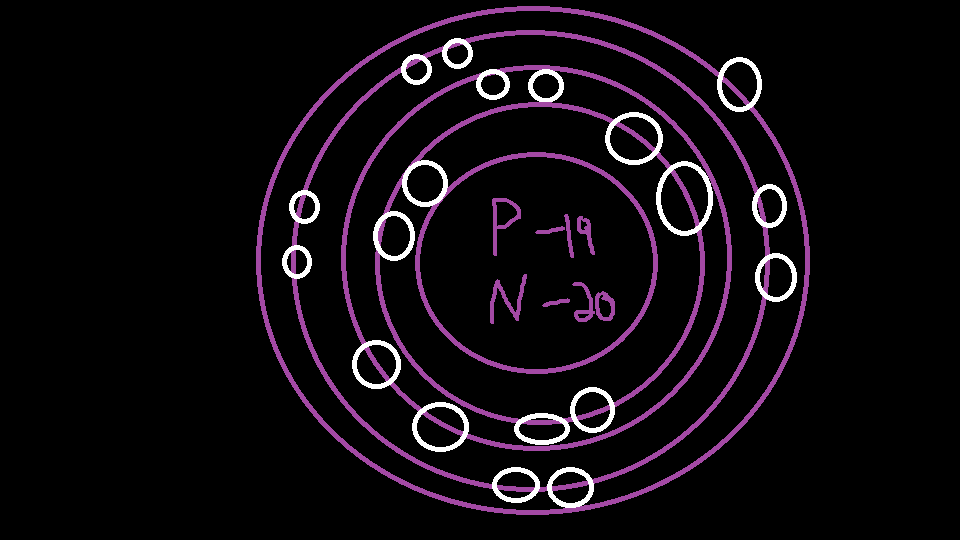
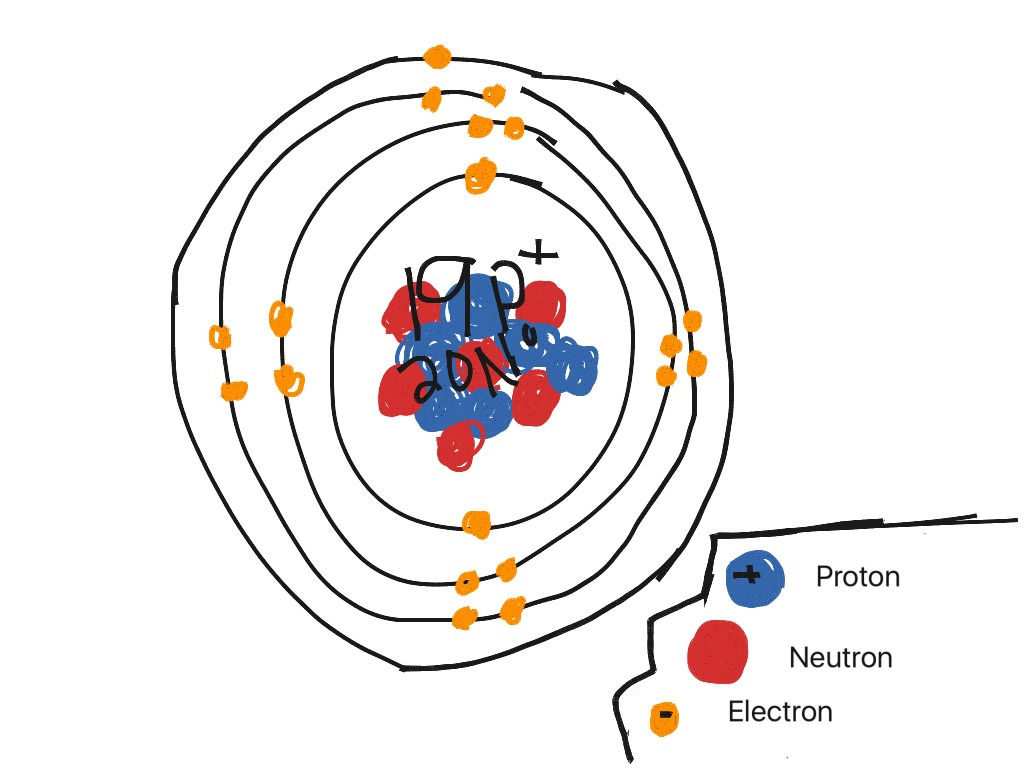
Steps Write protons, neutrons, and electrons of potassium atom Potassium has 19 protons, 20 neutrons, and 19 electrons. Learn how to find: Potassium protons neutrons electrons Draw nucleus of potassium atom The nucleus of a potassium atom contains 19 protons and 20 neutrons. So draw the nucleus of potassium atom as follows: Potassium nucleus